Current projects
1) Expression, regulation and structure/function relation of cationic amino acid transporters (CATs)2) Identification and characterization of the lysosomal transporter involved in cysteamine-mediated cystine efflux
3) Mechanisms of intracellular accumulation of the nitric oxide synthase (NOS) inhibitor asymmetrical L-Arginine (ADMA)
4) Synthesis, radiosynthesis and evaluation of dimeric amino acid chelator complexes for tumor diagnostic and PET
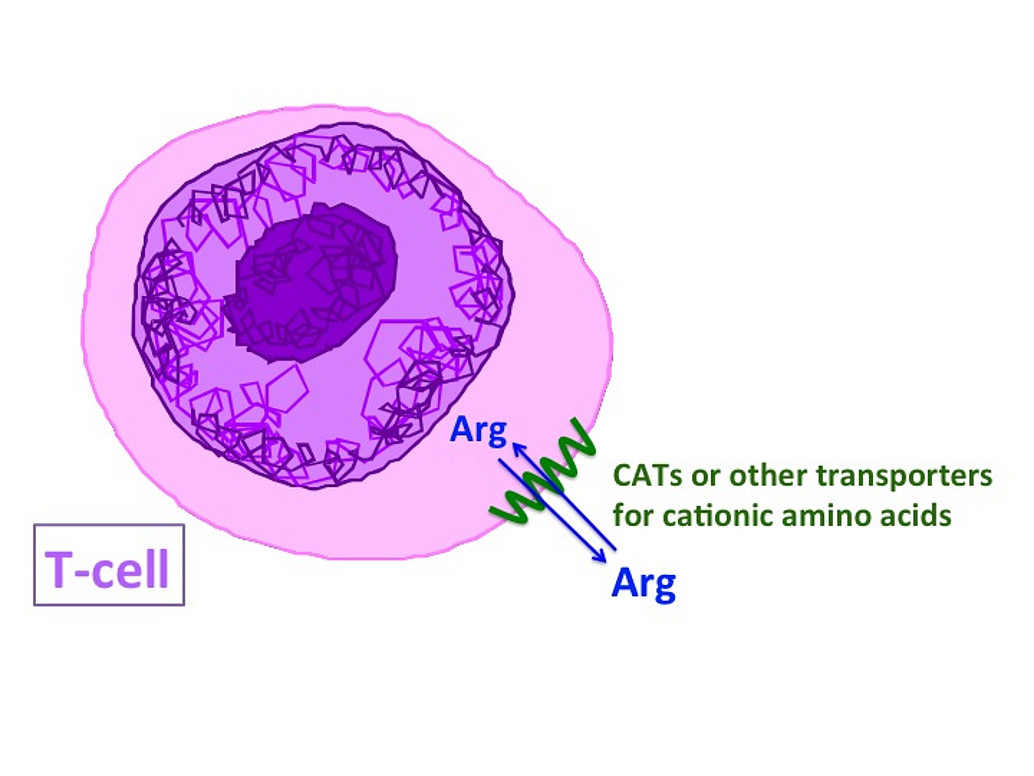
5) Regulation and role of arginine transport in human T-Lymphocytes.
Solute transport through biological membranes
Transport proteins in plasma or other biological membranes mediate the passage of charged, hydrophilic, or large substrates across these membranes, otherwise impassable for these molecules. They are hence crucial for virtually any cellular process involving such molecules. Transporters are therefore important drug targets. Many human diseases are caused by the absence or dysfunction of transporters. In addition, transporters attract interest due to their involvement in the uptake, metabolism and excretion of many drugs.CATs and HATs
Our group is mainly interested in the transport of cationic amino acids mediated by CATs (Cationic Amino acid Transporters) and HATs (Heteromeric Amino acid Transporters), both assigned to the gene family 7 of solute carriers (SLC7) by the Human Genome Organization. Cationic amino acids feed into protein synthesis, and other enzymatic reactions dependent on these amino acids, including the synthesis of nitric oxide (NO), urea, creatine and agmatine from arginine or the synthesis of polyamines, proline and glutamine from ornithine. Increasing evidence exists that CATs and HATs can be important determinants of these processes.1) Expression, regulation and structure/function relation of cationic amino acid transporters (CATs)
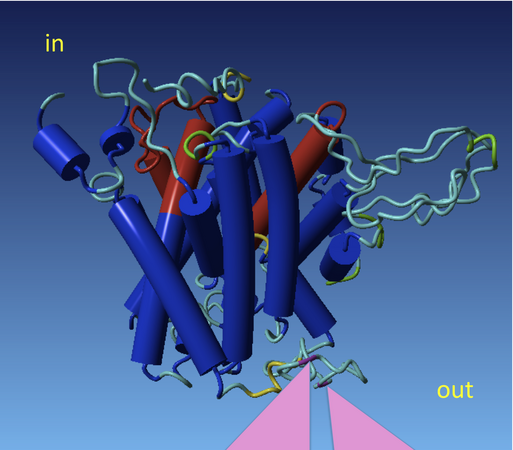
CAT proteins are plasma membrane transporters for cationic amino acids (for example arginine, lysine and ornithine) and thus important substrate providers for essential enzymatic processes such as the synthesis of nitric oxide, urea, polyamine, creatine, agmatine, proline and glutamate. Recent findings show in addition, that CAT proteins are involved in the mTor (mammalia target of rapamycin) pathway, a key regulator of many cellular processes. CATs form a subfamily of the solute carrier family 7 (SLC7) with four bona fide isoforms (CAT-1, -2A, -2B and -3) encoded by three different genes. Expression of the individual isoforms is cell type dependent and underlies complex regulation. For example, new mechanisms of translational control have recently been unraveled for both, CAT-1 and CAT-2 expression. The physiological function of each isoform demanding such a tight expressional control, is still largely unknown. In this project, we continue to investigate regulation and function of these proteins in order to better understand their physiological role and to detect ways to manipulate their activity.This work is funded by grant Cl 100/4-3 from the Deutsche Forschungsgemeinschaft (DFG)
hCAT1
2) Identification and characterization of the lysosomal transporter involved in cysteamine-mediated cystine efflux
Cystinosis is a rare genetic disorder that causes an accumulation of the amino acid cystine within cells, forming crystals that can build up and damage the cells. It is due to a mutation in the gene CTNS which codes for the lysosomal cystine transporter. The aminothiol cysteamine represents the only treatment of cystinotic patients to date. In the lysosomal lumen, cysteamine reacts with cystine to form a cysteine-cysteamine conjugate that (because of its structural similarity to lysine) leaves lysosomes through a transporter selective for cationic amino acids. The latter is known biochemically as lysosomal ‘system c’, but remains unknown at the molecular level. It represents a ‘salvage pathway’ which by-passes the need for a functional cystine transporter.In this project, we aim at identifying system c at the molecular level and developing a screening system for sulfhydryl compounds that act on cystinotic cells at lower concentration than cysteamine, thus providing a rationale approach to reduce the side-effects and constraints of the cysteamine treatment (gastrointestinal distress, administration every 6 hours).
Research in this project occurs in collaboration with Dr. Bruno Gasnier, Paris . It is funded by the Cystinosis Research Foundation, USA
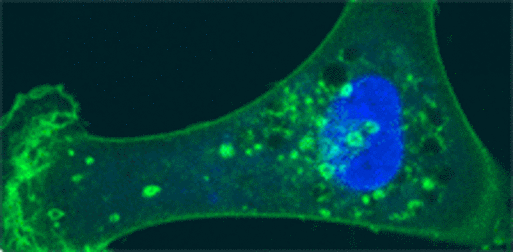
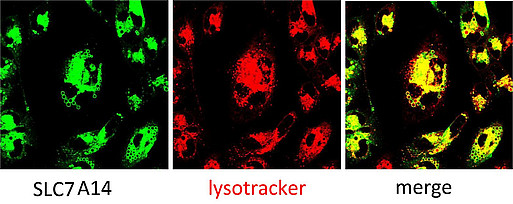
Cellular localization
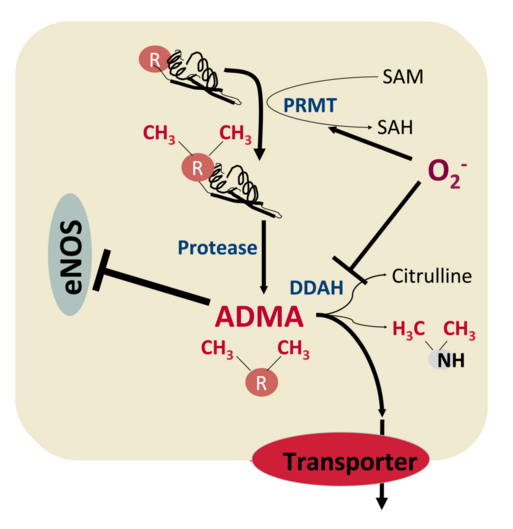
3) Mechanisms of intracellular accumulation of the nitric oxide synthase (NOS) inhibitor asymmetrical L-Arginine (ADMA)
NO, generated in both, endothelial cells and platelets, by the endothelial NO synthase (eNOS), reduces the activation, adhesion and aggregation of platelets. Furthermore, the recruitment of additional platelets to an established thrombus is constrained by NO. An absence of bioactive NO is associated with arterial thrombosis in animal models as well as in human individuals suffering from endothelial dysfunction.Our aim is to elucidate if the endogenous eNOS inhibitor asymmetric dimethylarginine (ADMA) accumulates as a result of reduced export and/or metabolism in endothelial cells and platelets. This hypothesis is based on the observation of an uncoupled eNOS (revealed through the production of superoxide radicals instead of NO) associated with a strongly reduced ADMA export in platelets from a patient. In addition, previous results show a considerable ADMA accumulation in endothelial cells under conditions not allowing ADMA efflux. We thus plan to investigate if ADMA accumulates in platelets under pro-atherothrombotic conditions and if this leads to uncoupling of eNOS. This approach will set the stage for further investigations of reduced ADMA export, platelet function and thrombus formation under experimental conditions. In a translational approach we will evaluate if we observe a reduced ADMA export in a larger number of patients and if this correlates with thrombotic events.
The focus of this project is on the role of transmembrane transport in the pathogenesis of thrombosis and hemostasis. Our objective is to identify ADMA export proteins and to resolve their function and regulation in thrombosis and hemostasis.
Funding: Project A4 - Center for Thrombosis and Haemostasis (CTH), Mainz.
Transporter-mediated efflux - a determinant of intracellular ADMA concentration?
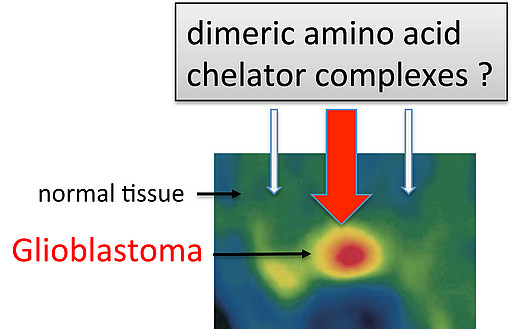
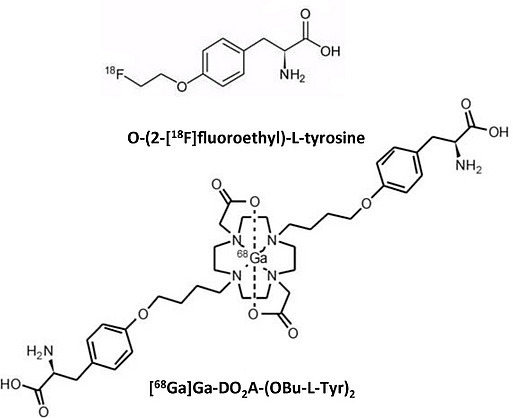
4) Synthesis, radiosynthesis and evaluation of dimeric amino acid chelator complexes for tumor diagnostic and PET
Glioblastoma is the most common and most aggressive type of primary brain tumor in humans. For the development of new effective treatment strategies, it is crucial to monitor tumor growth and spreading with sensitive markers. Labeled amino acids, such as 18F-tyrosine, are being used to detect tumors by positron emission tomography. Suitable 68Ga-labeled amino acids could have a high clinical relevance, because 68Ga can be generated by a 68Ge/68Ga-generator system and can thus be made available for routine diagnostic.The goal of this project is to synthesize and evaluate 68Ga-containing dimeric amino acid chelator complexes for tumor diagnostic and PET.
Our part is the identification of amino acid transporters in glioma cells that serve as docking station for the amino-acid-containing complexes. We further aim to elucidate the mechanism with which the complexes are internalized in tumor cells.
The project is a collaboration with Prof. Dr. Frank Rösch, Institute for Nuclear Chemistry, Johannes Gutenberg University, Mainz. It is funded by the DFG (our part: Cl 100/5-1)
Markely increased amino acid transport (FET-PET)
5) Regulation and role of arginine transport in human T-Lymphocytes.
The amino acid arginine is essential for the function of human T-lymphocytes. Arginine depletion during inflammatory reactions, as well as in tumor disease, leads to clinically relevant immune suppression and inhibition of the anti-tumor immune response, respectively. Arginine transport proteins, present in human T-lymphocytes, are therefore potential new targets for the pharmacological manipulation of T-cell functions.
In collaboration with PD Dr. Markus Munder (Medical University Mainz, Med III.: Hematology, Oncology and Pneumology), we will investigate the following central questions concerning arginine transport in this system:
(1) Which transport proteins mediate the uptake of the amino acid arginine in human T-lymphocytes at rest, and upon activation and cellular stress (arginine depletion)?
(2) What is the subcellular localization of these proteins and how are they regulated?
(3) What other transport proteins affect, directly or indirectly, the availability of arginine in human T-lymphocytes?
(4) Can the function of human T-lymphocytes be regulated by selective modulation of specific transport proteins for arginine?
In collaboration with PD Dr. Markus Munder (Medical University Mainz, Med III.: Hematology, Oncology and Pneumology), we will investigate the following central questions concerning arginine transport in this system:
(1) Which transport proteins mediate the uptake of the amino acid arginine in human T-lymphocytes at rest, and upon activation and cellular stress (arginine depletion)?
(2) What is the subcellular localization of these proteins and how are they regulated?
(3) What other transport proteins affect, directly or indirectly, the availability of arginine in human T-lymphocytes?
(4) Can the function of human T-lymphocytes be regulated by selective modulation of specific transport proteins for arginine?
This work is funded by grant CL 100/6-1 from the Deutsche Forschungsgemeinschaft (DFG): 2012-2015.