Methods
Standard and specific Molecular Biology’s methods (DNA, RNA, Protein) are currently utilized in our group, as for example: cloning, bacterial and mammalian DNA transfections, gene reporter assays, siRNA transfection, Real-Time qRT-PCR, immunoblotting (Western), antibody preparation, culture and biochemical analysis of cells, microscopy, live cell imaging, cell sorting, …
Rather than attempting to present a detailed, comprehensible list of all the protocols used in our lab, we will focused on some specific methods employed in ours projects:
Rather than attempting to present a detailed, comprehensible list of all the protocols used in our lab, we will focused on some specific methods employed in ours projects:
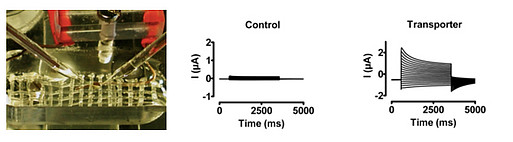
Transport Studies in Xenopus laevis Oocytes
Oocytes from the african frog Xenopus laevis provides a well suited expression system for the study of membrane transporters. cDNAs coding for various transporter proteins (wild types, mutants, chimeric EGFP-transporters, accessory chains, ...) can be transcribed in vitro, capped, poly-adenylated, and the resulting cRNA transferred into the oocyte's cytoplasma by microinjection. Following translation, proper folding, post-translational modifications, and sorting, fully functional expression of mature membrane proteins is achieved. Various biochemical analyses, such as transport activity assay, western, immunostaining, fluorescence microscopy, can be then conducted.

Sigel, E. (1990). Use of Xenopus oocytes for the functional expression of plasma membrane proteins. J Membr Biol 117, 201-221.
Vékony, N., Wolf, S., Boissel, J.-P., Gnauert, K., and Closs, E.I. (2001). Human cationic amino acid transporter hCAT-3 is preferentially expressed in peripheral tissues. Biochemistry 40, 12387-12394.
Brown, D.D. (2004). A tribute to the Xenopus laevis oocyte and egg. J Biol Chem 279, 45291-45299.
Bröer, S. (2010). Xenopus laevis Oocytes. Methods Mol Biol 637, 295-310.
Vékony, N., Wolf, S., Boissel, J.-P., Gnauert, K., and Closs, E.I. (2001). Human cationic amino acid transporter hCAT-3 is preferentially expressed in peripheral tissues. Biochemistry 40, 12387-12394.
Brown, D.D. (2004). A tribute to the Xenopus laevis oocyte and egg. J Biol Chem 279, 45291-45299.
Bröer, S. (2010). Xenopus laevis Oocytes. Methods Mol Biol 637, 295-310.
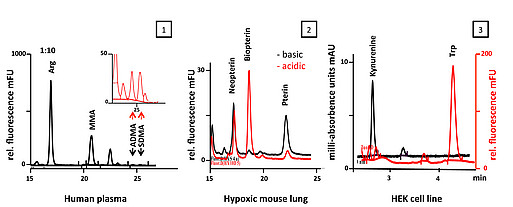
Quantification of Solutes by High Performance Liquid Chromatography (HPLC)
1. Determination of amino acid content (in plasma, urine, cell culture supernatants, cell line and tissue extracts) using automated pre-column OPA/ FMOC- derivatization and fluorescence detection.
Teerlink, T., Nijveldt, R.J., de Jong, S., and van Leeuwen, P.A.M. (2002). Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem 303, 131-137.
2. Measurement of Tetrahydrobiopterin (BH4), Dihydrobiopterin (BH2), and Biopterin after oxidation in lysates from tissues and cultured cells; detection by fluorescence.
Werner-Felmayer, G., and Gross,S:S, (1966). Analysis of tetrahydrobiopterin and its role in nitric oxide synthesis. In: M. Feelisch and J.S. Stamler, Editors, Methods in Nitric Oxide Research, John Wiley and Sons, New York (1966), pp. 271–299.
3. Measurement of Tryptophane and Kynurenine by simultaneous UV and fluorescence detections in serum and cell extracts.
Widner, B., Werner, E.R., Schennach, H., Wachter, H., and Fuchs, D. (1997). Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem 43, 2424-2426.
Teerlink, T., Nijveldt, R.J., de Jong, S., and van Leeuwen, P.A.M. (2002). Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem 303, 131-137.
2. Measurement of Tetrahydrobiopterin (BH4), Dihydrobiopterin (BH2), and Biopterin after oxidation in lysates from tissues and cultured cells; detection by fluorescence.
Werner-Felmayer, G., and Gross,S:S, (1966). Analysis of tetrahydrobiopterin and its role in nitric oxide synthesis. In: M. Feelisch and J.S. Stamler, Editors, Methods in Nitric Oxide Research, John Wiley and Sons, New York (1966), pp. 271–299.
3. Measurement of Tryptophane and Kynurenine by simultaneous UV and fluorescence detections in serum and cell extracts.
Widner, B., Werner, E.R., Schennach, H., Wachter, H., and Fuchs, D. (1997). Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem 43, 2424-2426.
NO Detection - RFL-6 Reporter Cell Assay
NO-mediated vasodilation mainly depends on nitrosylation of the heme-iron in soluble guanylate cyclase (sGC), resulting in enzyme activation and intracellular cGMP accumulation. By use of a reporter cell line with considerable amounts of sGC (Rat Lung Fibroblast cells, RFL-6), we can assay the short half-life NO by quantifying intracellular cGMP by ELISA.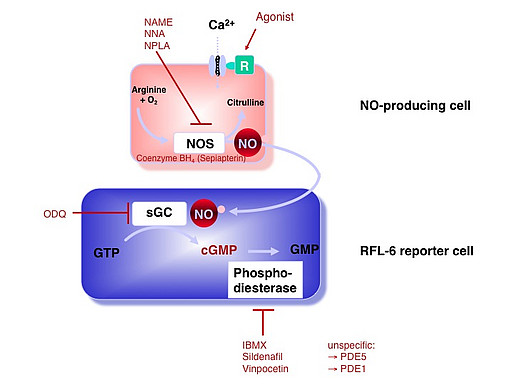
Ishii, K., Gorsky, L., Förstermann, U., and Murad, F. (1989). Endothelium-derived relaxing factor (EDRF): the endogenous activator of soluble guanylate cyclase in various types of cells. J App Cardiol 4, 505-512.
Förstermann, U., Closs, E.I., Pollock, J.S., Nakane, M., Schwarz, P., Gath, I., and Kleinert, H. (1994). Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23, 1121-1131.
Förstermann, U., Closs, E.I., Pollock, J.S., Nakane, M., Schwarz, P., Gath, I., and Kleinert, H. (1994). Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23, 1121-1131.