Research Group Adult Neurogenesis and Cellular Reprogramming
Our research focuses one side on the molecular and cellular mechanisms underlying physiological adult neurogenesis while addressing on the other the possibility of inducing via cellular reprogramming de novo genesis of neurons in brain areas that are naturally devoid of neurogenesis.
Lineage Progression of Adult Neural Stem Cells
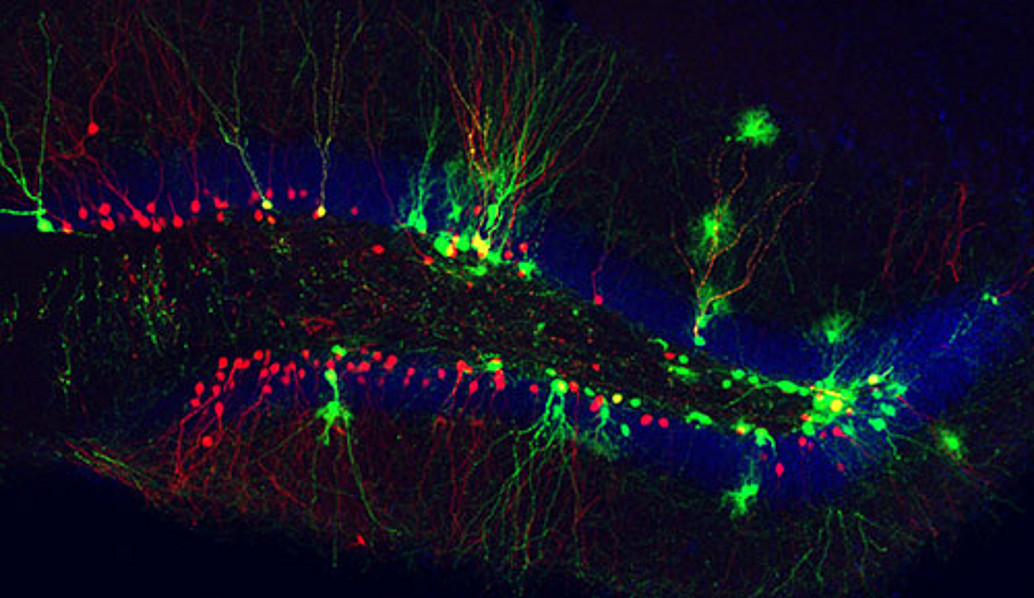
Two regions in the mammalian brain are capable of life-long production of new neurons and glia: the subgranular zone (located right under the granule cell layer of the dentate gyrus of the hippocampus) and the subventricular zone bordering the lateral ventricle. Both regions harbor neural stem cells capable of self-renewal as well of producing progeny that differentiates into distinct types of neurons and glia. The latter comprise oligodendroglia which is responsible for the formation of the myelin sheaths enwrapping nerve fibers that degenerate in diseases like multiple sclerosis. We investigate the mode of cell division of neural stem cells and the mechanisms that regulate the decision whether a undifferentiated cell gives rise to neuron or glia (Costa et al., 2011; Ortega et al., 2011). A key question which we study intensely concerns the issue whether one and the same neural stem cell generates both neuron and oligodendroglia. By means of time-lapse videomicroscopy we were able to show that adult subventricular zone neural stem cells in vitro produce either neuronal or oligodendroglial progeny, but never both (Ortega et al., 2013). Moreover, we found that the generation of new oligodendroglia can be selectively enhanced upon stimulation of the Wnt signaling pathway. These findings were fully corroborated in the adult subventricular zone in vivo. This data provide important evidence for the possibility of selectively enhancing oligodendrogliogenesis, thereby opening eventually new avenues for the treatment of diseases like multiple sclerosis.
http://www.youtube.com/watch?v=I2uu8oFTl7w
The video shows the lineage progression of a single neural stem cell isolated from the adult subventricular Zone.
The video shows the lineage progression of a single neural stem cell isolated from the adult subventricular Zone.
Integration of Adult-Generated Neurons into the Pre-existing Network
Another theme of our research concerns the question how newly generated neurons integrate into a pre-existing neural network. Surprisingly, many adult-generated neurons die soon after their birth (for review see (Bergami and Berninger, 2012)). To better understand the process of incorporation of adult-generated neurons into the neuronal circuitry we developed a method which allows for following the establishment of synaptic contacts onto newly generated neurons in vivo over time (Deshpande et al., 2013). Neurons generated in the subgranular zone migrate over short distance into the granular layer where they differentiate in granule neurons. With the help of a modified rabies virus we can examine how the innervation of newly generated granule neurons by their presynaptic partners evolves over time. Using this approach we found that 1-2 weeks after their birth new neurons receive synaptic contacts from neurons of their immediate vicinity, i.e., local interneurons. Only 1-2 weeks later synaptic inputs from more distant brain areas arrive, such as cholinergic input from the medial septum. Finally, only at the end of their maturation process (3-5 weeks) new granule neurons become innervated by pyramidal neurons in the entorhinal cortex and thus form part of the classical trisynaptic hippocampal circuit. The changes in the innervation pattern suggest that during the course of their maturation newly generated neurons exert distinct influences on hippocampal information processing.