Behl group the autophagy lab mainz
Biochemistry of Neurodegeneration and Aging
The most frequent human neurodegenerative disorders including Alzheimer’s Disease, Parkinson’s Disease and amyotrophic lateral sclerosis are strictly associated with age; to decode the molecular mechanisms underlying neurodegeneration we therefore start by analyzing the molecular differences of young and aged neurons. In particular, we focus on cellular protein homeostasis (proteostasis) – its importance being underlined by the occurrence of specific protein aggregates in several age-associated neurodegenerative disorders– as well as on adaptive mechanisms neurons employ to compensate and adapt to different forms of cellular stress.Proteostasis and autophagy
The maintenance of protein homeostasis – a balance between synthesis, folding and controlled degradation of proteins – is of vital importance for all cellular functions. Misfolded, damaged, dysfunctional or aggregated proteins can be degraded and removed via two different mechanisms: the ubiquitin proteasome system and autophagy. In the course of macro¬autophagy intracellular material (protein aggregates, organelles, pathogens etc.) is gradually engulfed by a membrane which closes at last to form the autophagosome. After fusing with lysosomes its content is hydrolytically degraded. The specificity of the degradation-prone material is ensured by receptor molecules that interact with the substrate as well as with protein components of the membrane (selective macroautophagy). On one hand, macro¬autophagy is of pivotal importance for cellular (protein) quality control and stress response but serves to recycle cell components and to produce energy in the case of nutritional deprivation on the other.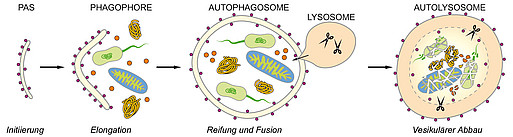
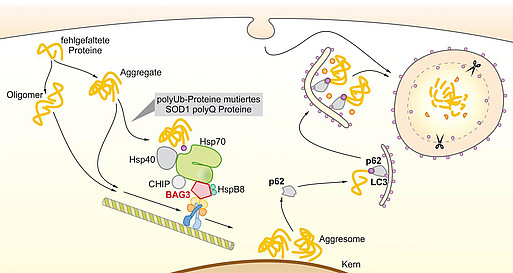
In 2009 we described for the first time a selective macroautophagy pathway driven by the HSP70 co-chaperone BAG3 (BCL-2 athanogene 3). This BAG3-mediated selective macroautophagy is used as cells face acute proteotoxic stress or the oxidative, proteotoxic environment that comes along with cell aging, and is capable to guide disease-associated aggregating proteins for degradation. Currently, we are analyzing the kinetics of this process, the regulation of BAG3-mediated selective macroautophagy by endo- and exogenous factors and post-translational modifications and its interplay with other autophagy pathways within the Collaborative Research Centre 1177 (CRC 1177 Molecular and functional characterization of selective autophagy).
To identify so far unknown factors of proteostasis we employ specific C. elegans (worm) lines and RNAi libraries. By an RNAi screen we were able to characterize the RAB3GAP complex as a novel positive modulator of autophagy that takes part in the formation of autophagosomes. In subsequent studies we were able to show that this activity is mediated by the RAB GTPase RAB18, a component of the cellular vesicular transport system. Mutations of the genes coding for RAB3GAP1, RAB3GAP2 and RAB18 cause the Warburg Micro syndrome, an autosomal recessive disease characterized by severe neuronal impairment during early development. One goal of our research efforts is to clarify the interplay between autophagy and RAB18 as well as the mobilization of lipids in neurons.
Another gene identified via the C.elegans RNAi screen as novel component of the cellular proteostasis network is RME8 (receptor mediated endocytosis 8; human ortholog: DNAJC13) that belongs to the HSP40 protein family and is characterized by the binding to HSP70. RME8 through its interaction with the retromer complex is part of the endosomal transport system. We were able to show that RME8 is also a positive modulator of autophagy. Currently, we are working on RME8 function possibly as a link between the endosomal transport system and the maintenance of proteostasis. A particular focus is put here on the analysis of a mutant RME8 variant associated with familial forms of Parkinson’s Disease. The role of RME8 especially in neuronal cells is studied within the Collaborative Research Centre 1080 (CRC 1080 Molecular and cellular mechanisms of neuronal homeostasis).
Another gene identified via the C.elegans RNAi screen as novel component of the cellular proteostasis network is RME8 (receptor mediated endocytosis 8; human ortholog: DNAJC13) that belongs to the HSP40 protein family and is characterized by the binding to HSP70. RME8 through its interaction with the retromer complex is part of the endosomal transport system. We were able to show that RME8 is also a positive modulator of autophagy. Currently, we are working on RME8 function possibly as a link between the endosomal transport system and the maintenance of proteostasis. A particular focus is put here on the analysis of a mutant RME8 variant associated with familial forms of Parkinson’s Disease. The role of RME8 especially in neuronal cells is studied within the Collaborative Research Centre 1080 (CRC 1080 Molecular and cellular mechanisms of neuronal homeostasis).
Adaptation of neurons to cellular stress
We are investigating the ability of cells to adapt to internal and external stress factors playing an important role under physiological aging conditions as well as in the pathogenesis of neurodegenerative disorders. Interestingly neuronal stress can adapt to an extreme pro-oxidative milieu and loose the sensitivity to oxidative stress. During this adaptation process an increased autophagic activity appears to play a role.
In another project part we aim to clarify the cellular processes that are activated or inhibited by pathophysiological conditions, such as oxidative stress, reduced perfusion of the cells (as manifested by nutrient and oxygen reduction and mitochondria dysfunction) and how they contribute to neurodegeneration. Moreover, we are investigating which metabolic mechanisms increase the adaptation potential of neurons. Therefore, in the focus of these projects is the analysis of the modulation of autophagy, of changes in signal transduction components as well as the role of mitochondria. Since mitochondria have a key role in aging and neurodegeneration, we aim to find out which role the perturbation of mitochondrial synthesis, mitochondrial dynamics and mitochondrial degradation (mitophagy) plays.
One strategy for neuroprotection that we are following is the use of pharmacological antioxidants to prevent oxidative damage. Moreover, we aim to develop novel therapeutic approaches that contribute to an increased neuronal adaptation to nutrition and oxygen deprivation to prevent pathophysiological consequences of hypoperfusion. To analyse functional alterations at the biochemical and molecular level in neurons we in parallel evaluate synaptic neurotransmission via field potential recordings and patch clamp technique. These projects are funded by the Corona Foundation.