Evolutionary Biochemistry and Redox Medicine
Univ.-Prof. Dr. rer. nat. Bernd Moosmann
We are interested in the fundamentals and the evolution of modern redox biochemistry. On a molecular level, evolution has shaped all biochemical pathways and structures including those related to human disease and aging. The elucidation of causal factors that have molded modern biochemistry might thus be of unique value for the understanding of disease-causing processes. Specifically, we study neurodegenerative disorders, metabolic disease, and the general biological aging process.
Our projects:
1. Neurodegeneration and Aging
Redox dysregulation and oxidative stress are a characteristic of neurodegenerative disorders and appear to be pervasive in the biological aging process. However, it is unsettled whether redox alterations are indeed causally relevant originators of malfunction, or whether they rather reflect secondary signs of an already ongoing degenerative process. If aberrant oxidation had a causal role in diseases like Alzheimer’s disease, specific redox-modulatory compounds could be of substantial therapeutic value.
Aiming at a deeper understanding of disease-relevant redox processes, we work on a systematic characterization of macromolecular damage in the young, old and diseased mammalian brain. In parallel lines of investigation, we try to clarify the potential role of usage-dependent and early developmental processes as upstream triggers of age-related neurodegeneration. To study the basic biological aging process, we employ invertebrate in vivo models including nematodes and fruit flies.
-
Sohre S, Moosmann B (2018). The pathological hallmarks of Alzheimer’s disease derive from compensatory responses to NMDA receptor insufficiency. BioRxiv 418566.
-
Kunath S, Moosmann B (2020). What is the rate-limiting step towards aging? - Chemical reaction kinetics might reconcile contradictory observations in experimental aging research. Geroscience 42, 857-866.
-
Kunath S, Schindeldecker M, De Giacomo A, Meyer T, Sohre S, Hajieva P, von Schacky C, Urban J, Moosmann B (2020). Prooxidative chain transfer activity by thiol groups in biological systems. Redox Biol. 36, 101628.
-
Moosmann B (2021). Flux control in the aging cascade. Aging 13, 6233-6235.
-
Moosmann B, Hajieva P (2022). Probing the role of cysteine thiyl radicals in biology: eminently dangerous, difficult to scavenge. Antioxidants 11, 885.
Oskar Fischer Prize for Bernd Moosmann
More on the Oskar Fischer Prize for Alzheimer's disease research from UTSA
2. Proteomic Biochemistry and the Origins of Life
Impressive amounts of molecular information on all forms of life have been collected by the DNA sequencing initiatives launched in the last two decades. Unfortunately, the functional interpretation of those data arrays is severely lagging behind the sequencing efforts that continue to generate an increasingly complete genomic picture of life.
Employing comparative bioinformatics approaches on the proteome and genome level as well as quantum chemical methods, we aspire to detect general rules of biochemical evolution that have shaped modern life during its billion-year history. Specifically, we try to better understand the evolution of the genetic code and the origins of chemodiversity, especially related to the vast number of secondary metabolites found in microbial and plant communities. Our results may one day become useful for the detection of life on other planets.
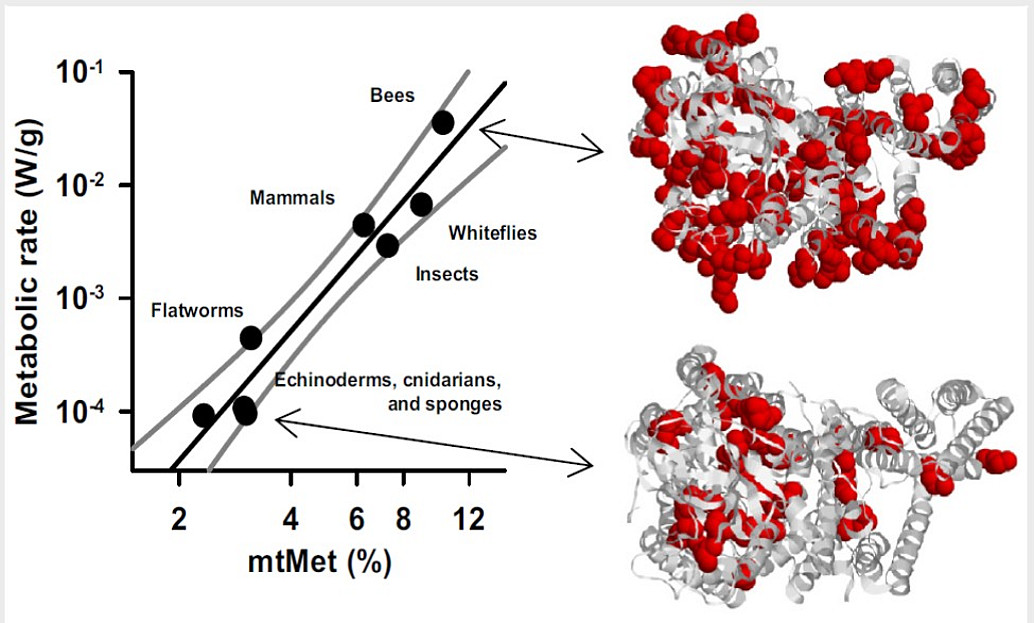
-
Granold M, Hajieva P, Tosa M, Irimie FD, Moosmann B (2018). Modern diversification of the amino acid repertoire driven by oxygen. Proc. Natl. Acad. Sci. USA 115, 41-46.
-
Moosmann B (2021). Redox biochemistry of the genetic code. Trends Biochem. Sci. 46, 83-86.
-
Schindeldecker M, Moosmann B (2024). Cysteine is the only universally affected and disfavored proteomic amino acid under oxidative conditions in animals. Antioxidants 13, 267.
-
Abrosimov R, Moosmann B (2024). The HOMO-LUMO gap as discriminator of biotic from abiotic chemistries. Life 14, 1330.
3. Regulation of Redox Metabolism
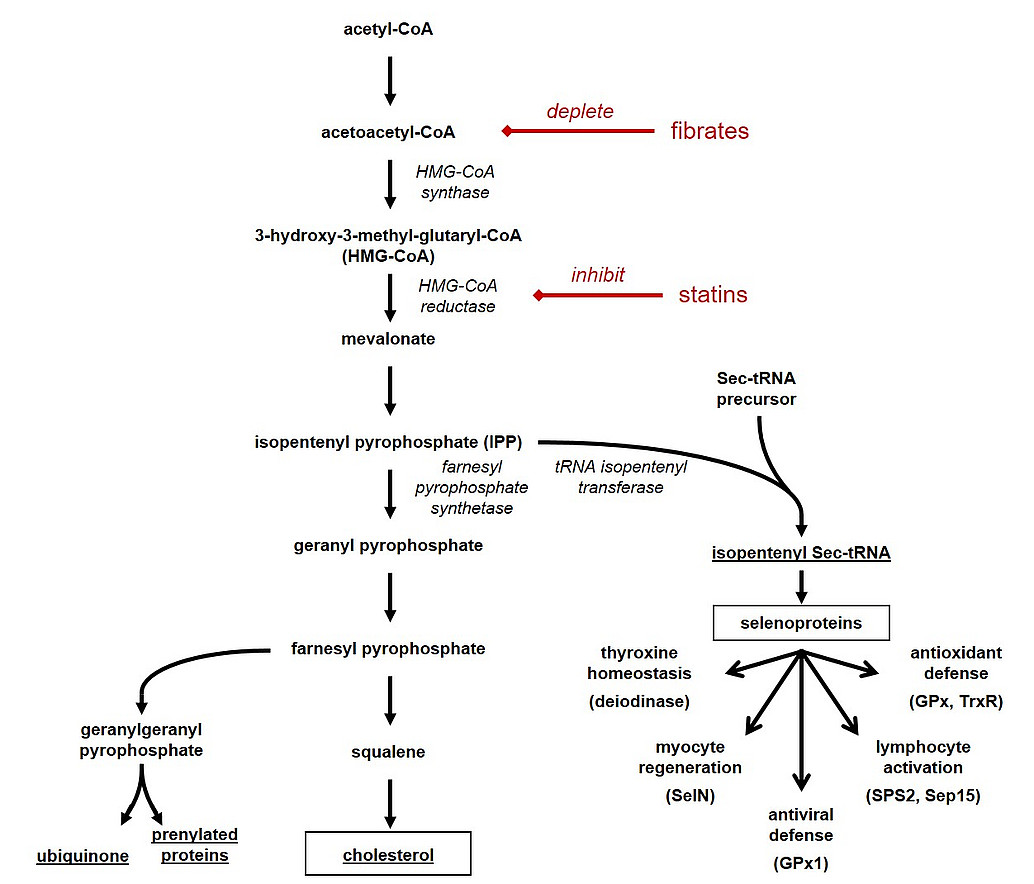
Beyond its essential role as a membrane component and metabolic precursor to various steroid hormones, cholesterol has long been recognized as a prooxidant effector molecule in vivo. Potentially, this effect is causal to the apparent association of cholesterol with many diseases including atherosclerosis. Paradoxically, cholesterol-lowering drugs (commonly known as statins) that inhibit endogenous cholesterol biosynthesis can exert both prooxidant as well as antioxidant effects, entirely depending on the precise model or patient cohort studied. We hypothesize that a tissue-specific modulation of antioxidant selenoprotein expression might be critically involved in the potent redox effects of cholesterol modulation. We are currently investigating this idea in different genetic and nutritional models.
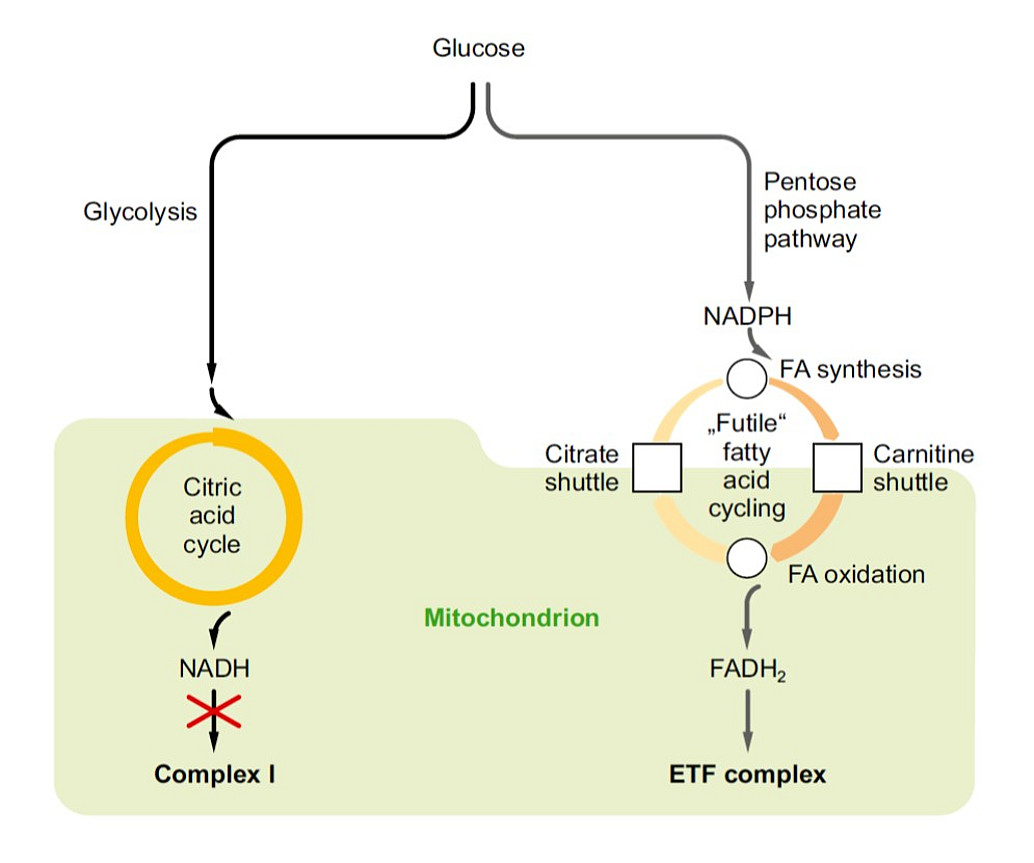
Various unrelated antidiabetic interventions such as methionine restriction, metformin administration or fibrate/thiazolidinedione treatment share respiratory chain complex I inhibition as direct or indirect executive mechanism. However, there is as yet no satisfying consensus answer to the question why and how complex I inhibition counters the development type II diabetes. In analyzing metabolic crosstalk and transcriptomic changes following complex I inhibition in different experimental systems, we strive to solve this riddle.
-
Kromer A, Moosmann B (2009). Statin-induced liver injury involves cross-talk between cholesterol and selenoprotein biosynthetic pathways. Mol. Pharmacol. 75, 1421-1429.
-
Fuhrmeister J, Tews M, Kromer A, Moosmann B (2012). Prooxidative toxicity and selenoprotein suppression by cerivastatin in muscle cells. Toxicol. Lett. 215, 219-227.
-
Baeken MW, Kötzner P, Richly H, Behl C, Moosmann B, Hajieva P (2023). Adaptive epigenetic regulation of neuronal metabolism by a mitochondrial redox signal. BioRxiv 570533.
-
Abrosimov R, Baeken MW, Hauf S, Wittig I, Hajieva P, Perrone CE, Moosmann B (2024). Mitochondrial complex I inhibition triggers NAD+-independent glucose oxidation via successive NADPH formation, “futile” fatty acid cycling, and FADH2 oxidation. Geroscience 46, 3635-3658.