Forschung
Early Cortical Activity
Early development of mammalian brains can be functionally characterized by the progressive emergence of distinct cortical activity patterns. The emergence of correlated oscillatory activity patterns, such as spindle burst and gamma oscillations in newborn rodents or delta brushes in prenatal human, coincidences with the maturation of many essential anatomical and functional neuronal correlates.
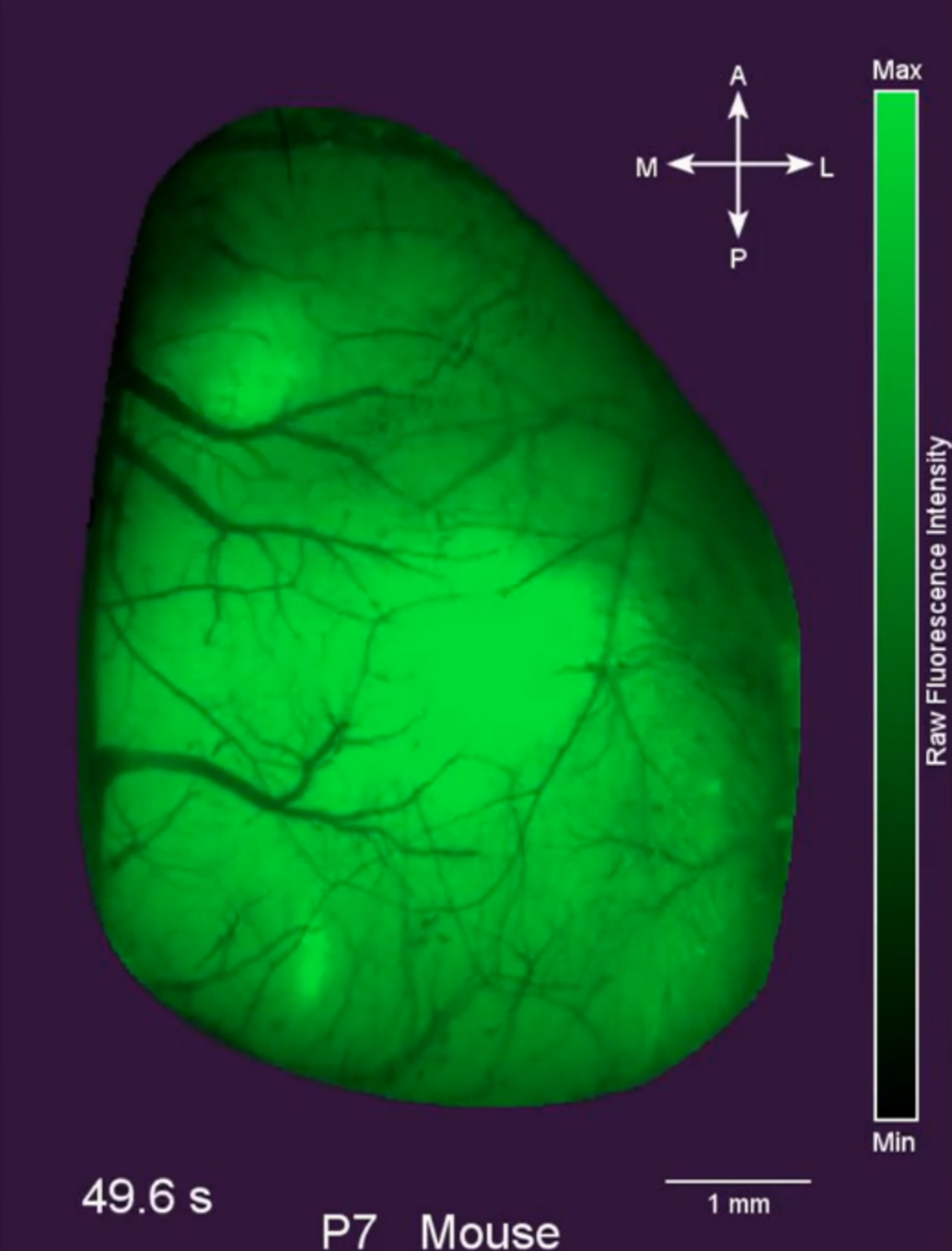
Adapted from Warm et al., 2025
Consequently, the cerebral cortex can generate local and coherent patterns of activation, which allow the brain to operate through the functional segregation and hierarchical integration of neuronal modules. Thereby it can process the different sensory inputs and coordinate them with motor control to orchestrate behavior. Hence, it is not surprising, that the absence of certain activity patterns during critical developmental periods is associated with unfavorable outcomes in humans and animal models.
In our current and future experimental studies, we investigate how early activity patterns emerge and interact within large-scale cortical dynamics in order to better understand physiological development of the cortex. Since pathological causes, e.g. hypoxia, stroke, or pharmacological treatments, can strongly affect early cortical activity, we are also studying cause and consequence of such activity alterations using different models in vivo and in vitro.
Selected publications
Warm D, Bassetti D, Gellèrt L, Yang JW, Luhmann HJ, Sinning A (2025). Spontaneous mesoscale calcium dynamics reflect the development of the modular functional architecture of the mouse cerebral cortex. Neuroimage. 2025 Feb 13:121088. doi: 10.1016/j.neuroimage.2025.121088.
Warm D, Schroer J, Sinning A* (2022) Gabaergic Interneurons in Early Brain Development: Conducting and Orchestrated by Cortical Network Activity. Front Mol Neurosci. 14:807969. doi: 10.3389/fnmol.2021.807969
Luhmann HJ, Sinning A, Yang JW, Reyes-Puerta V, Stüttgen MC, Kirischuk S, Kilb W (2016) Spontaneous Neuronal Activity in Developing Neocortical Networks: From Single Cells to Large-Scale Interactions. Front Neural Circuits. 2016 May 24;10:40. doi: 10.3389/fncir.2016.00040
Developmental Apoptosis
Apoptosis, programmed cell death, occurs throughout life of all species and also widely affects the nervous system during embryonic and early postnatal periods as part of its physiological development. Besides genetic programs and neurotrophic factors, electrical activity and synaptic inputs directly regulate neuronal survival in the central nervous system. As a result, alterations of apoptotic rates during critical phases of development are associated with abnormal cortical circuits and impaired functionality of the brain. But also, alteration in neuronal activity levels lead to pathological changes in apoptotic rates and thus to abnormal neuronal population sizes. Within developing cortical networks survival or apoptotic removal of single neurons can be reliably predicted based on its prior activity profile. In line, apoptotic rates can be modulated by optogenetic manipulation of neuronal firing pattern. Thus, activity is a pro-survival factor; however, how this activity-dependent effect is translated into enhanced survival chances on a neuronal level is not fully understood.
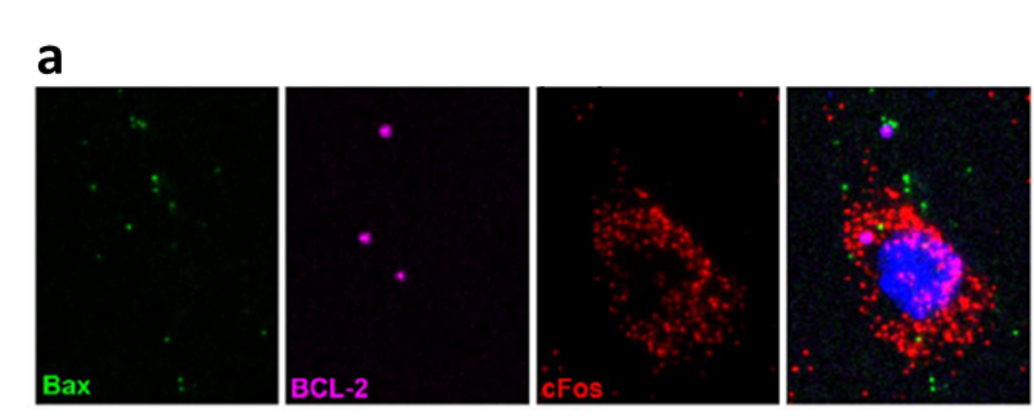
Adapted from Schroer et al., 2023
In a recent work of our group we could, for example, show that electrical activity reduces the relative expression of key players in the mitochondrial apoptosis pathway (BAX and BCL-2) in immature neurons. In this way tolerance for apoptotic CASP3 activity is selectively raised in active neurons and thus results in a higher likelihood of survival during early cortical development.
In our current and future studies we are addressing the local and global effects of local manipulation of early cortical activity on the regulation of apoptotic processes. Also, we address the neuroprotective effect of high-frequency, oscillatory neuronal activity and its underlying cellular and molecular transitions during critical phases of development.
Selected publications
Schroer J, Warm D, De Rosa F, Luhmann HJ, Sinning A* (2023) Activity-dependent regulation of the BAX/BCL-2 pathway protects cortical neurons from apoptotic death during early development. Cell Mol Life Sci. 2023 Jun 3;80(6):175. doi: 10.1007/s00018-023-04824-6.
Wong Fong Sang E, Schroer J, Halbhuber L, Warm D, Yang JW, Luhmann HJ, Kilb W, Sinning A* (2021) Optogenetically controlled activity pattern determines survival rate of developing neocortical neurons. Int J Mol Sci. 22(12):6575. doi: 10.3390/ijms22126575
Blanquie O, Yang JW, Kilb W, Sharopov S, Sinning A*, Luhmann HJ* (2017) Electrical activity controls area-specific expression of neuronal apoptosis in the mouse developing cerebral cortex. Elife. 21;6. pii: e27696. doi: 10.7554/eLife.27696
GABAergic integration of neuronal populations
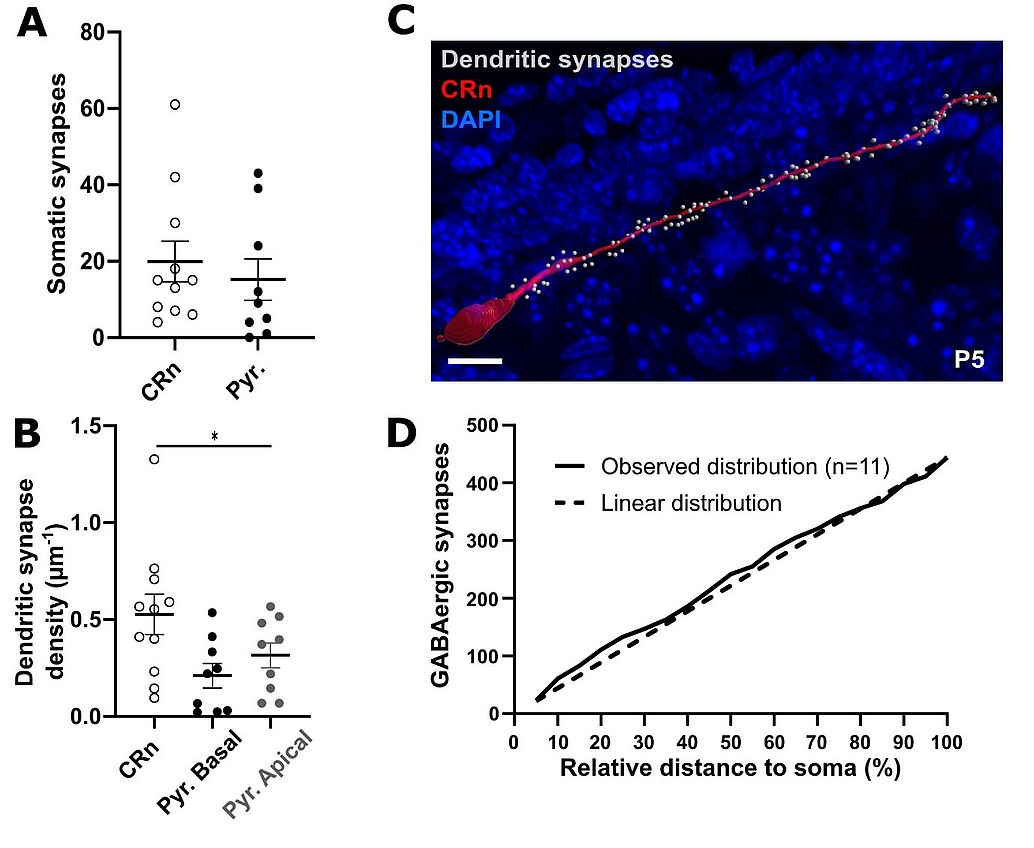
Adapted from Abusaada et al., 2025
Activity-dependent maturation of the GABAergic system promotes the desynchronization of network activity and thus underlies cortical dynamics during early development. During the perinatal period, GABAergic inputs are especially prominent in transient neuronal population of Cajal-Retzius neurons, which amongst other functions guide cortical layering by the release of Reelin. Gradually, the various types of GABAergic interneurons, which populate the mature cortex with its diverse functions, are integrated into the maturing cortical network. However, to date, surprisingly little information is available on the time course, with which cortical GABAergic synapses are formed. However, the gradual integration of interneurons premises the controlled formation of GABAergic synapses within a dynamically changing landscape of participant neurons in the cortex by largely unknown mechanisms. For Cajal-Retzius neurons we could previously show that a novel developmental death pathway mediated by NKCC1, via GABAA receptor-mediated membrane depolarization and p75NTR signaling in CRNs controls apoptosis of CRNs (Blanquie et al 2017). Interestingly a more recent study of our group revealed that Cajal-Retzius neurons indeed represent a major target of very early GABAergic synapses in the marginal zone with a homogeneous synapse distribution along the dendrite (see Figure).
In our current and future studies, we address the largely unknown mechanisms by which GABAergic neurons are integrated into the dynamically changing cortical landscape, i.e. when and where GABAergic synapses are formed. We are especially interested in the early GABAergic integration of distinct cortical subpopulations, e.g. Cajal-Retzius neurons and how this integration contributes to immature cortical activity patterns and the structural and functional maturation of the brain.
Selected publications
Abusaada A, De Rosa F, Luhmann HJ, Kilb W* and Sinning A* (2025). GABAergic integration of transient and persistent neurons in the developing mouse somatosensory cortex. Front Cell Neurosci doi: 10.3389/fncel.2025.1556174
Blanquie O, Lutz Liebmann, Christian A. Hübner, Luhmann HJ*, Sinning A* (2016) NKCC1-mediated GABAergic signaling promotes postnatal cell death in neocortical Cajal-Retzius cells. Cereb Cortex. doi: 10.1093/cercor/bhw004
Methods
- Organotypic and dissociated cortical models
- Multi-electrode-recordings and optical imaging in vito
- Wide-field calcium imaging and extracellular recordings in vivo
- AAV-based overexpression
- Optical Imaging and optogenetics
- Immunhistochemistry (antibody stainings, tissue clearing) and molecular biology (realtime PCR, multiplexed fluorescence in situ hybridization, Western Blot etc.)
We happily accept applications for master thesis and medical doctorates !
Wir freuen uns über Bewerbungen für Masterarbeiten, sowie für (medizinische) Doktorarbeiten !
