TRP X35 Megakaryocyte transcriptome profiling upon alteration of key platelet regulators
Funding period: 01.08.2019 – 31.07.2020
Project Summary
Beside their well-established roles in thrombosis and hemostasis, platelets also participate in an increasing variety of biological processes, like inflammation and tumor cell metastasis. Despite being anucleated, their specific function depends on platelet regulators expressed on their surface. As a result, platelets have to be equipped with the necessary mRNA and proteins during thrombopoiesis from megakaryocytes (MKs), their parental cells.
MKs are large, granular, polyploid cells and derive from hematopoietic stem cells (HSCs). After induction, bone marrow HSCs differentiate into immature MKs. During maturation, MKs become polyploid by endomitosis and their cytoplasm increases in size, reaching diameters of 60 μm and ploidy of 128N. Furthermore, this process is associated with an evolutionary acquisition of functional receptors, necessary for different platelet functions. Accordingly, the MKs transmigrate to the bone marrow sinusoids, form multiple proplatelets and equip them with different types of RNA transcripts, as well as RNA processing machinery and different proteins. The complete process is called megakaryopoiesis. Pathological megakaryopoiesis is often associated with thrombocytopenia and can be due to a defect in the functionality of platelet regulators, like GPIb or von Willebrand Factor (VWF).
Indeed, MKs also express platelet regulators, and they were historically thought to be preferentially stored intracellularly in the α-granules. However, recent research demonstrates that platelet receptors like TLT-1, VWF, or GPIb actually translocate to the MK surface. Moreover, it was shown that alterations of the interaction between GPIb and VWF induce pathological platelet production, and is associated with von Willebrand disease type2B (VWD). Therefore, a compelling open question is related to whether and how the expression of key platelet regulators defines the MK function by acting on their heterogeneity, both in physiological and in disease contexts.
To answer this question, we will use different mouse models, i.e. TLT-1 knockdown (KD) and GPIb-IL4 chimera (expressing a nonfunctional GPIb), and analyze alterations in their transcriptome. TLT-1 is a transmembrane receptor stored in the α-granules of platelets. Upon thrombin-induced platelet activation, TLT-1 is transported to the platelet surface, where it is cleaved and can bind its ligands, fibrinogen and VWF. Furthermore, preliminary data from the Ruf Lab show an upregulation of TLT-1 in platelets from tumor-bearing mice, as well as in the recovery phase of thrombopoiesis after depletion. GPIb is a MK-specific mechanoreceptor, which coordinates thrombopoiesis. Together with GPIX and GPV, GPIb forms the VWF receptor complex, and defects in this complex account for Bernard-Soulier syndrome (BSS) and a macrothrombocytopenia. Since both proteins, TLT-1 and GPIb, are receptors for VWF and regulate thrombopoiesis, a common signalling pathway might be involved in these processes.
The study of MKs has proven to be challenging, since they are fragile, relative rare, and tend to aggregate. Consequently, highly selective and innovative methods are needed for successful isolation of MKs and the definition of their transcriptomes.
We plan to isolate MKs from TLT-1 KD and GPIb/IL4 chimera strains as well as the corresponding wild type animals; moreover, we will use genetically modified induced pluripotent stem cell (iPSC) derived human MKs to perform bulk RNA-seq and identify and compare KD dependent alteration in the MK transcriptome in mouse and human. Furthermore, we will use single cell sequencing to unmask heterogeneity within the MK population (see scheme in Fig. 1). We expect to gain a deeper understanding of VWF regulation, unraveling new insights related to pathological megakaryopoiesis.
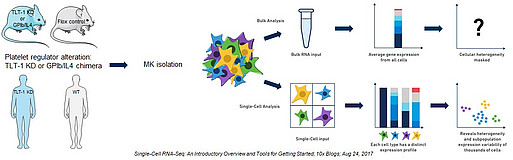
Figure 1: Workflow of the TRP Project. Murine TLT-1 KD or GPIb/IL4 chimera and wt MKs as well as human iPSC derived TLT-1 Kd and wt MKs will be isolated. Extracted RNA will be used for bulk and single cell transcriptome analysis, performed on both the gene and the transcript level.
Principal Investigators
