Immunotherapy
Aktuelle Projekte der wissenschaftlichen Arbeitsgruppen des Kinderonkologischen Zentrums konzentrieren sich daher auf die Anpassung von Immuntherapien an die pädiatrische Population, einschließlich der Aufklärung von Resistenzmechanismen und die Integration dieser Ansätze in die Therapie pädiatrischer Krebserkrankungen.
New Project: CAR-T cells therapy for solid pediatric tumors
Immunotherapy using CAR-T cells targeting GD2 has shown remarkable success in treating high-risk neuroblastoma. However, applying this strategy to other solid tumors remains challenging due to the scarcity of tumor-specific targets with minimal expression on healthy tissues. A major concern is the risk of CAR-T cells cross-reacting with normal tissues, leading to severe toxicity from on-target/off-tumor effects. Therefore, identifying targets that are absent from vital normal tissues is essential for safe and effective therapy. In this project, we aim to discover and characterize surface proteins and lipids that can serve as novel targets in various pediatric tumor types. One promising candidate is Claudin 6 (CLDN6), an oncofetal tight junction protein expressed during embryonic epithelial development but largely absent from healthy adult tissues, except for the placenta. Importantly, CLDN6 expression is reactivated in several tumors. This has spurred the development of CLDN6-specific CAR-T cells and bispecific T cell-engaging antibodies, currently being evaluated in basket clinical trials involving adult patients. Ultimately, our goal is to pave the way for including pediatric patients in CAR-T cell studies targeting such novel antigens.
Project relevant publication:
Seidmann L, Wingerter A, Oliver Metzig M, Bornas A, El Malki K, Ustjanzew A, Ortmüller F, Kamyshanskiy Y, Kindler T, Laible M, Mohr X, Henninger N, Russo A, Beck O, Alt F, Wehling P, Roth W, Paret C, Faber J. The Chimeric Antigen Receptor T Cell Target Claudin 6 Is a Marker for Early Organ-Specific Epithelial Progenitors and Is Expressed in Some Pediatric Solid Tumor Entities. Cancers (Basel). 2025 Mar 7;17(6):920. doi: 10.3390/cancers17060920. PMID: 40149257; PMCID: PMC11940025.
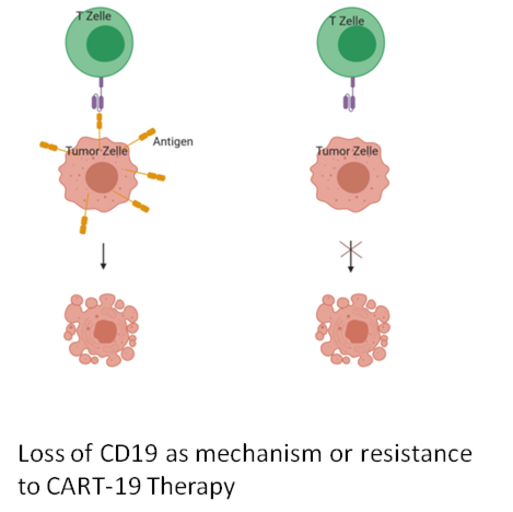
Project 1. Mechanisms of resistance to CART-19 therapies
Relapsed and chemotherapy-refractory B-cell acute lymphoblastic leukaemias (B-ALL) remain significant causes of cancer-associated morbidity and mortality for children. An emerging immunotherapy uses modified T-cells expressing chimeric antigen receptors (CART) against CD19 (CART-19) to treat children with high-risk B-ALL. In CART-19 therapy, T cells of the patient are engineered to express CARTs that link a single-chain anti-CD19 antibody to the intracellular signalling domain of the T-cell receptor. Upon CD19 recognition, the CARTs activate the cytotoxic T-cells to attack the tumour cells. CART-19 therapy was recently approved for the treatment of paediatric B-ALL in the USA and Europe. Unfortunately, about 10-20% of children relapse under CART-19 therapy. Up to 50% of these therapy-resistant cases were characterised by the loss of detectable CD19 epitope. In this project, we want to identify predictive biomarkers for the escape from CART-19 therapy and to develop strategies to avoid the loss of the CD19 target epitope.
Project relevant publications:
Fischer J, Paret C, El Malki K, Alt F, Wingerter A, Neu MA, Kron B, Russo A, Lehmann N, Roth L, Fehr EM, Attig S, Hohberger A, Kindler T, Faber J. CD19 Isoforms Enabling Resistance to CART-19 Immunotherapy Are Expressed in B-ALL Patients at Initial Diagnosis. J Immunother. 2017 Jun;40(5):187-195. doi:
10.1097/CJI.0000000000000169. PMID: 28441264; PMCID: PMC5424577.
High-throughput mutagenesis identifies mutations and RNA-binding proteins controlling CD19 splicing and CART-19 therapy resistance. Cortés-López M, Schulz L, Enculescu M, Paret C, Spiekermann B, Quesnel-Vallières M, Torres-Diz M, Unic S, Busch A, Orekhova A, Kuban M, Mesitov M, Mulorz MM, Shraim R, Kielisch F, Faber J, Barash Y, Thomas-Tikhonenko A, Zarnack K, Legewie S, König J. Nat Commun. 2022 Sep 22;13(1):5570. doi: 10.1038/s41467-022-31818-y. PMID: 36138008 Ziegler N, Cortés-López M, Alt F, Sprang M, Ustjanzew A, Lehmann N, El Malki K, Wingerter A, Russo A, Beck O, Attig S, Roth L, König J, Paret C, Faber J. Analysis of RBP expression and binding sites identifies PTBP1 as a regulator of CD19 expression in B-ALL. Oncoimmunology. 2023 Mar 1;12(1):2184143. doi: 10.1080/2162402X.2023.2184143. PMID: 36875548; PMCID: PMC9980455.
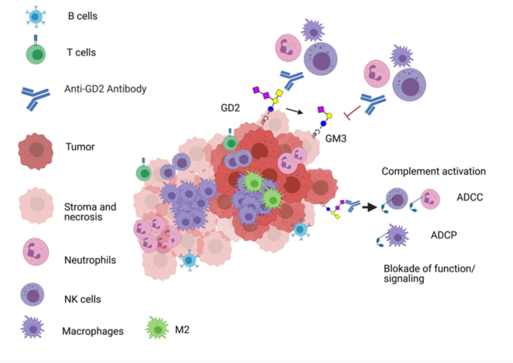
Project 2. Gangliosides as targets in pediatric tumors
Project 2. GD2 and Gangliosides as targets in pediatric tumors
Gangliosides are sialylated glycosphingolipids composed of ceramide and an oligosaccharide complex and are found in the plasma membrane. Some gangliosides, such as GD2, have a low expression in normal tissues but are overexpressed in several tumor entities. Due to this restricted expression, GD2 has long been recognized as an important target for cancer immunotherapy . However, the anti-GD2 antibodies dinutuximab and naxitamab are released only for the treatment of children with high-risk neuroblastoma so far. We have recently shown that GD2 is expressed also by other pediatric tumor entities and that GD2 is a relevant target not only for immunotherapies but also for targeted therapies. In this project, we will analyse the expression, regulation and druggability of GD2 and other gangliosides in pediatric tumors. Moreover, we have developed an assay for the detection of GD2 by mass spectrometry in tumor tissues. This assay is currently used for the enrollment of GD2 positive Ewing Sarcoma in the CESS-GD2 clinical study (NCT06839703). The assay will be also adapted to detect GD2 in liquid biopsy.
Project 3. Relevance of immune checkpoint inhibitors in paediatric tumours
Contact: Dr. med. Khalifa El Malki, TransMed Fellowship
Project 4. Non conventional T cells as new players in cancer immunotherapy
Project 4. The Immune Microenvironment of Pediatric Tumors
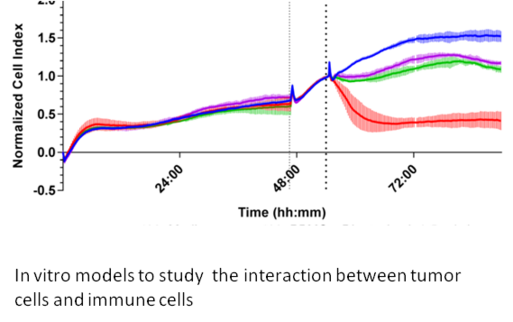
Immune cells within the tumor microenvironment play a pivotal role in regulating tumor growth and shaping responses to immunotherapy. Effector cells such as cytotoxic T lymphocytes (CD8+ T cells) and natural killer (NK) cells can directly attack and eliminate tumor cells. In contrast, immunosuppressive populations—including regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs)—promote tumor survival by dampening anti-tumor immune responses. The dynamic balance and functional state of these immune cells critically influence the immune system’s ability to recognize and eradicate cancer. Understanding this complex immune landscape is essential for developing and optimizing immunotherapies tailored to pediatric cancers, ultimately improving treatment outcomes and patient survival.
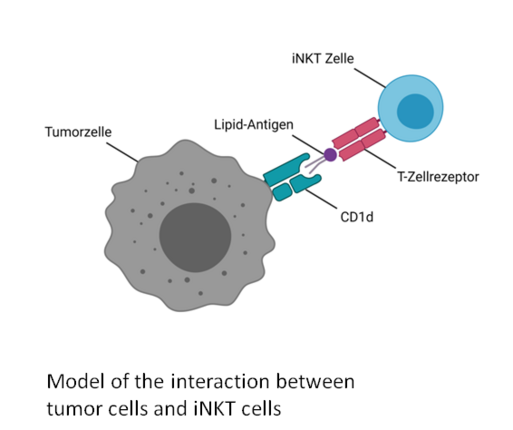
While most current immunotherapies focus on harnessing the anti-tumor activity of conventional CD8+ cytotoxic T cells, recent research highlights the potential of nonconventional T cells—such as invariant natural killer T (iNKT) cells and γδ T cells—to recognize and eliminate tumor cells. Unlike conventional αβ T cells, iNKT and γδ T cells recognize antigens independently of HLA haplotypes, offering promising avenues for broadly applicable, pan-population cancer immunotherapies. Specifically, iNKT and Vδ1 T cells detect lipid antigens presented by CD1d molecules; however, the exact nature of these lipid ligands in pediatric tumors remains largely unknown.
In this project, we aim to analyze the infiltration of both conventional and nonconventional T cells in pediatric tumors, identify endogenous lipid ligands that activate these cells, and investigate how gangliosides shed by tumor cells influence the function of cytotoxic immune cells. We are using the Akoya Akoya Phenocyclers for high-plex spatial biology and tissue imaging to analyse the composition of the Immune Microenvironment.