Platform 8
Vascular Remodeling
The rupture of an atherosclerotic plaque with subsequent thrombosis and tissue perfusion reduction in distal regions is a frequent complication within the cardiovascular system and the cause underlying clinical syndromes, such as myocardial infarction, stroke or peripheral-arterial ischemia. If the acute event is survived, wound healing processes are activated, which contribute to the restoration of vascular integrity and perfusion, but also may be disturbed (e.g. in the presence of cardiovascular risk factors). To examine the pathophysiology of vascular repair processes, specialized mouse models have been developed, which allow the detailed analyses of the cellular, structural and molecular processes as well as of critical mediators or therapeutic strategies under standardized conditions.
Models and Methods
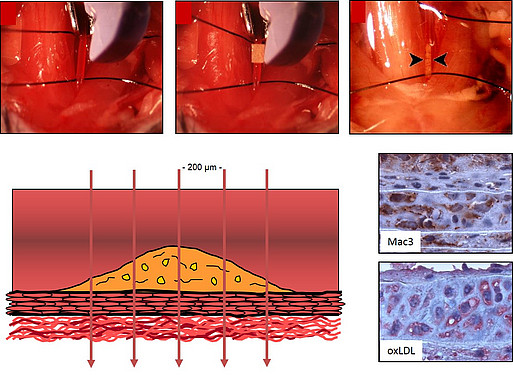
The Platform laboratory „Vascular Wound healing“ offers the following methods to analyze vascular repair processes following arterial thrombosis or ischemia:- Morphometric analysis of neointima formation (restenosis) following ferric chloride-induced thrombosis at the A. carotis communis or of atherosclerotic lesions (at the aortic root or the A. brachiocephalica) in the mouse
- Protocols for the immunohistochemical analysis of the cellular and structural composition of vascular lesions
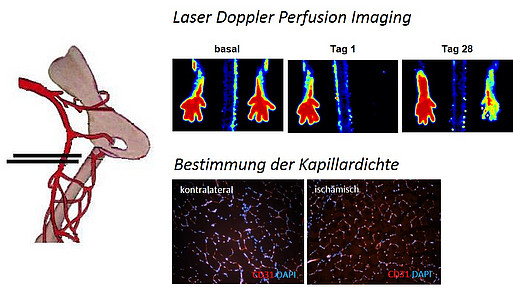
- Non-invasive quantification of vascular reperfusion (using Laser Doppler Perfusion Imaging) following the induction of unilateral hindlimb ischemia in the mouse
- Quantification of neovascularization (angio- and arteriogenesis) in vivo, ex vivo and in vitro (e.g. Matrigel™- and spheroid angiogenesis assay)
Mouse model of arterial thrombosis and neointima formation using ferric chloride (FeCl3)
Mouse model of unilateral hindlimb ischemia to study neovascularization
Completeted cooperations
- Chrysanthopoulou A, Kambas K, Stakos D, Mitroulis I, Mitsios A, Vidali V, Angelidou I, Bochenek M, Arelaki S, Arampatzioglou A, Galani IE, Skendros P, Couladouros EA, Konstantinides S, Andreakos E, Schäfer K, Ritis K. Interferon lambda1/IL-29 and inorganic polyphosphate are novel regulators of neutrophil-driven thromboinflammation. J Pathol. 2017 Sep;243(1):111-122.
- Luther N, Brähler M, Krebs F, Jäckel S, Subramaniam S, Stanger C, Schönfelder T, Reinhardt C, Wenzel P, Schäfer K, Becker C. Innate effector-memory T cell activation regulates post-thrombotic vein wall inflammation and thrombus resolution. Circ Res. 2016;119:1286-1295.
- Karbach S, Schönfelder T, Brandao I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schäfer K, Münzel T, Reinhardt C, Wenzel P. Gut microbiota promote Angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016; Aug 30;5(9). pii: e003698.
- Ben-Zvi D, Savion N, Kolodgie F, Simon A, Fisch S, Schäfer K, Bachner-Hinenzon N, Cao X, Gertler A, Solomon G, Kachel E, Raanani E, Lavi Y, Emeth SK, Virmani R, Schoen F, Schneiderman J. Local leptin antagonist attenuates angiotensin II-induced ascending aortic aneurysm. J Am Heart Assoc. 2016; May 3;5(5). pii: e003474.
Scientific Platform Coordinator

Professor Katrin Schäfer, MD
Head of Laboratory for „Translational Vascular Biology“, Department Cariology and Angiology / Center for Thrombosis and Hemostasis
