TRP X36 Integrated multi-omics analyses elucidating the interplay of coagulation and inflammation in the development and progression of type 2 diabetes mellitus
Funding period: 01.08.2019 – 31.07.2020
Project Summary
The prevalence of Type 2 Diabetes Mellitus (T2DM) has been rapidly increasing in most countries worldwide, representing a substantial threat to human health and a burden to public health systems. The situation is further aggravated by a potentially high proportion of undiagnosed prevalent T2DM cases, influenced e.g. by differences in progression, slow onset of often diffuse symptoms, or underestimation of the actual individual diabetes risk.
Moreover, the utility of current screening modalities for diabetes based on glucose and Glycated hemoglobin (HbA1c) appears to be limited in the view of complex heterogeneities in the progression to overt diabetes, caused by diverse pathogenic contributions, and in the clinical presentation of T2DM. As a result, an increasing number of newly defined diabetes subgroups, extending the classical subdivision into type 1 and type 2, manifests.
Amongst the pathogenic contributions, metabolic dysregulations comprise a cluster of conditions including dyslipidemia, altered proinsulin processing by pancreatic beta cells, hyperinsulinemia, insulin resistance, and impaired glucose homeostasis. All of these conditions are associated with inflammation and also related to platelet hyper-reactivity or hypercoagulation.
One important cell type in this regard is the monocyte, due to its role in chronic inflammation, which is driven for instance by advanced glycation end products or modified lipoproteins. Furthermore, monocytes (differentiating to macrophages) play an important role in obesity-induced, insulin resistance-associated inflammation of the adipose tissue and skeletal muscle.
Interestingly, monocytes have also been implicated as mediator linking insulin resistance to thrombotic complications, since insulin resistant monocytes have increased tissue factor expression, which is regarded as central trigger of coagulation and thrombus formation.
In this study, we aim to further elucidate the inflammation- and coagulation-related molecular changes during diabetes development and progression using a bioinformatics approach, and to link these molecular changes to differential expression in monocytes. We use data from the population-based Gutenberg Health Study (GHS) at baseline, including targeted proteomics data of 92 inflammation related proteins, microarray-based transcriptomics data of monocytes, thrombin generation curves and activation of coagulation factors, as well as detailed clinical data. Furthermore, we link these data to clinical information about diabetes development and (subclinical) cardiovascular disease at 5 years follow-up.
For analysis of this unique dataset, we use a bioinformatics approach including the detection of protein and transcript clusters with different expression patterns during diabetes development (Figure 1), pathway and network analysis of these clusters, identification of subgroups of individuals with different disease development paths, and characterization of these subgroups based on clinical data at baseline as well as disease development and clinical outcome during 5 years follow-up.
This novel approach for an extended molecular subtyping of individuals during diabetes development has direct translational potential, since subgroups with distinct diabetes development and varying underlying mechanisms could benefit from different disease prevention and treatment strategies.
Furthermore, elucidation of the link between diabetes and cardiovascular disease with a focus on inflammation and coagulation can provide novel insights into underlying mechanisms that can pave the way for development of new therapeutic approaches.
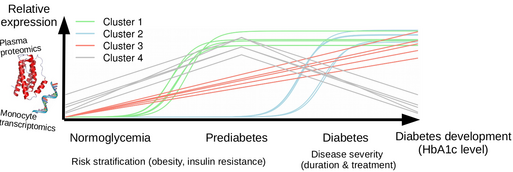
Figure 1: Schematic visualization of protein and transcript clusters with different patterns of change during diabetes development
Principal Investigator

Professor Philipp Wild, MD, MSc
Preventive Cardiology and Medical Prevention, Professorship „Clinical Epidemiology“, Coordinator Gutenberg Health Study (GHS), Principal Investigator Deutsches Zentrum für Herz-Kreislaufforschung (DZHK)
Staff
Christine Volkmar (Data management/Statistics)
Bianca Wagner (PhD candidate)